The Research Administration Portal “RAP” is now available for submissions. Please review the recording of the demo here.
All UAHS submissions involving patients, facilities, services, health records, and/or resources of a clinical provider (e.g., Banner Health/BH) must be submitted to our office here prior to submission to the University of Arizona Institutional Review Board (IRB). For new studies, the RAP initiates multiple processes including Banner feasibility review, coverage analysis development, budget & contract negotiations, financial review of ICF, and entering the study into the OnCore clinical trial management system (CTMS), as applicable.
Research Administration Portal
Does my study require a RAP submission?
If your study has a UAHS PI or requires the use of Banner resources, new and amended studies must be submitted to Research Administration using the RAP forms.
I’m not using Banner resources, why do I need to submit to the RAP?
For new studies, the RAP initiates multiple processes including Banner feasibility review, coverage analysis development, budget & contract negotiations, financial review of ICF, and entering the study into the OnCore (CTMS), as applicable.
New to RAP? No need to struggle through the process! We have experienced experts to help, please contact our office by email or schedule a consultation on MS Bookings, and we will guide you through the process.
For information regarding additional approvals that may be required prior to IRB submission, please review the links below:
-
Banner Health Research Feasibility Review
Required for all protocols that involve research being conducted at a BH site, BH services, patients with a treating relationship with BH (past/present), data or specimen collection, etc. -
UA Radiation Safety Review Committee
All protocols that involve exposing individuals to ionizing radiation for research purposes require UA Radiation Safety Committee review. Examples include: x-ray, PET, CT, DXA, nuclear medicine, and radiation therapy (e.g., radiation oncology studies). -
UA Institutional Biosafety Review Committee
See the NIH Guidelines for research that requires UA Institutional Biosafety Committee review. -
UA Human Subjects Protection Program (HSPP)
Our team can help with completion of the required forms for the IRB.
Frequently Asked Questions
Table of Content
- Is there training provided for the RAP? Who should attend?
- What documents are required for your project submission?
- Will study amendments be submitted to the RAP?
- Can I submit my Clinical Trial Agreement (CTA) to the RAP?
- I work in another College outside UAHS. Is the RAP for me?
- Does the research team submit directly to the IRB, Institutional Biosafety Review Committee, and/or Radiation Safety Review Committee?
- Where can I submit to the IRB?
- What happens once my project is submitted to the RAP?
- How do we track the progress of our research project?
Is there training provided for the RAP? Who should attend?
Yes, upon request UAHS Research Administration is happy to provide individual training and support with the RAP. Please email CRC@arizona.edu to schedule a training.
What documents are required for your project submission?
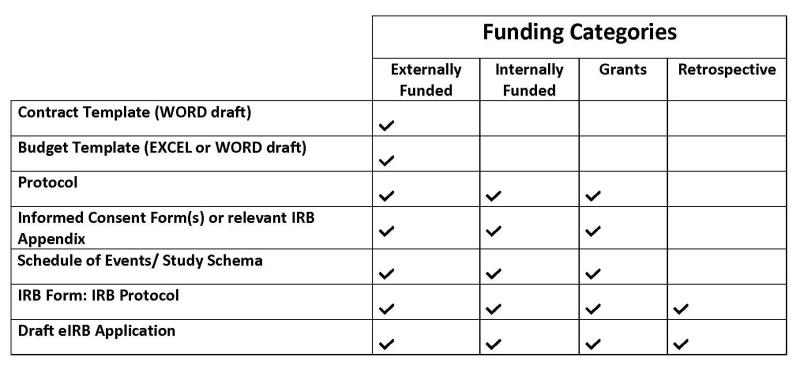
Will study amendments be submitted to the RAP?
Yes, a submission form for Amendments is now available for the following documents:
- Protocol Amendments
- Contract (CTA) Agreements
- Budget Amendments
- Revised Consent Forms
If your RAP Amendment application involves a CTA Amendment, the associated contracting amendment documents should be submitted.
If a Protocol Amendment affects the Schedule of Events (SOE), a revised SOE will need to be uploaded with the amedment application, so that the Coverage Analysis (CA) and Budget can be reviewed and updated for OnCore study calandar updates and billing compliance. Amendments should be submitted to the RAP as soon as possible so updates can be addressed concurrently with IRB review.
Can I submit my Clinical Trial Agreement (CTA) to the RAP?
Yes, the UAHS Research Administration Intake Team will route the agreement to the contract negotiator after feasibility approval. Contact CRC@arizona.edu with questions about the clinical trial agreement process.
I work in another College outside UAHS. Is the RAP for me?
Yes, if you are using Banner Health resources (including facilities, data, or employees), the RAP submission must be completed.
Does the research team submit directly to the IRB, Institutional Biosafety Review Committee, and/or Radiation Safety Review Committee?
Yes, you should submit directly to these committees (links are provided above).
Where can I submit to the IRB?
Studies that require the use of Banner resources, must receive their notice of feasibility approval prior to submission to the IRB. Funded studies must wait until the coverage analysis is complete and the project has been routed and approved through sponsored projects for submission to the IRB. This deferment in UA eIRB submission will eliminate duplicate COI disclosure requests and streamline consent form revisions.
What happens once my project is submitted to the RAP?
Below is a brief outline the UAHS process. Please feel free to contact us at any time if you are ever have any questions regarding your research project (CRC@arizona.edu).
- Application submission reviewed for completeness and sent to Banner Health Clinical Research Program Directors (CRPD) for feasibility approval.
- Project is reviewed and approved for feasibility by Clinical Research Program Directors (CRPDs).
- Your non-funded project can now be submitted to the IRB with a copy of your feasibility approval email.
- Contract is sent to UAHS Contracts for negotiations.
- Coverage Analysis (CA) development and submission to medical partner for approval.
- CA receives approval, internal proposal is routed, budget development begins.
- Internal proposal is approved by sponsored projects; funded projects can now be submitted to the IRB with a copy of your feasibility approval email.
- Budget and payment terms are negotiated.
- Final budget & payment terms are sent to UAHS Contracts for inclusion into contract.
- Contract negotiations finalized.
- Contract, inclusive of budget and payment terms, is routed for signatures.
- Contract finalized and fully executed.
- UAHS Research Administration will send a final packet to the PI & study team including the final contract, budget, and CA. UAHS Research Administration Oncore team will build the study calendar into the OnCore CTMS (Clinical Trial Management System) and notify study team when calendar is active.
How do we track the progress of our research project?
Please contact our office at CRC@arizona.edu for updates about submissions pending feasibility approval.
After feasibility review has been approved, project status can be obtained by reviewing the Contract Status Report. This report is updated every Monday and requires a NetID login to access.
Contact the IRB at VPR-IRB@arizona.edu directly with any questions regarding your IRB submission and the approval status, as they can provide the most complete and accurate information.

